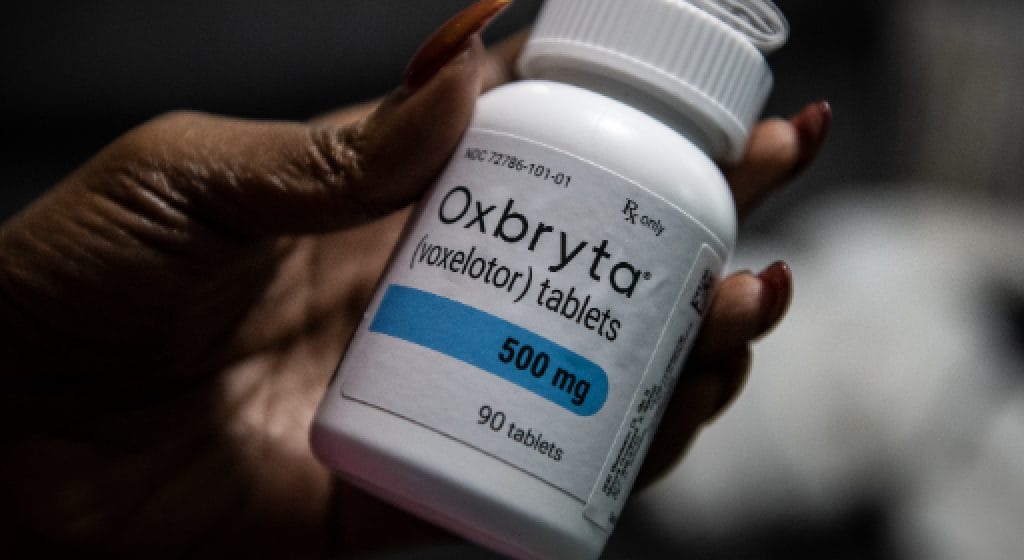
Oxbryta (voxelotor), a medication developed to treat sickle cell disease (SCD), has recently become the focus of significant legal action following its global recall in September 2024. Manufactured by Pfizer, Oxbryta was initially hailed as a breakthrough treatment for SCD, a genetic disorder characterized by abnormal hemoglobin that leads to distorted, sickle-shaped red blood cells. However, emerging safety concerns have led to a wave of lawsuits alleging severe side effects and inadequate warnings provided to patients and healthcare providers.
Background on Oxbryta
Approved by the U.S. Food and Drug Administration (FDA) in 2019 under its Accelerated Approval Program, Oxbryta was designed to inhibit hemoglobin polymerization, thereby reducing the formation of sickle-shaped cells and improving oxygen delivery. Despite its promising mechanism of action, post-marketing studies and adverse event reports have raised serious safety concerns.
Recent Developments in Oxbryta Litigation
As of December 2024, several key developments have unfolded in the ongoing Oxbryta litigation:
• Voluntary Market Withdrawal: On September 25, 2024, Pfizer announced a global recall of Oxbryta, citing that “the overall benefit of Oxbryta no longer outweighs the risk in the approved sickle cell patient population.” This decision was influenced by data indicating an increased risk of severe adverse events, including vaso-occlusive crises (VOCs), stroke, organ failure, and death among patients using the medication.
• Emergence of Lawsuits: Following the recall, numerous lawsuits have been filed against Pfizer and its subsidiary, Global Blood Therapeutics. Plaintiffs allege that the companies failed to adequately warn about the potential risks associated with Oxbryta, leading to severe health complications. For instance, an Illinois man filed a lawsuit in November 2024 after experiencing serious injuries attributed to Oxbryta.
• Regulatory Actions: The European Medicines Agency (EMA) recommended suspending the marketing authorization for Oxbryta as a precaution while a review of emerging data is ongoing. This aligns with Pfizer’s decision to withdraw the drug from the market, reflecting global concerns about its safety profile.
Implications for Affected Patients
Patients who have experienced adverse effects after using Oxbryta may be entitled to compensation for medical expenses, pain and suffering, lost wages, and other related damages. Legal experts suggest that settlement amounts could be substantial, especially in cases involving severe injuries or fatalities. Factors influencing settlement amounts include the severity of the injury, the impact on the patient’s quality of life, and the degree of negligence attributed to the manufacturer.
Legal Considerations for Potential Claimants
Individuals considering legal action should be aware of the following:
• Statute of Limitations: The timeframe to file a lawsuit varies by jurisdiction. It’s crucial to consult with an attorney promptly to ensure compliance with relevant deadlines.
• Documentation: Maintaining comprehensive medical records, including documentation of Oxbryta usage and any adverse effects experienced, is essential to support legal claims.
• Legal Representation: Engaging with a law firm experienced in pharmaceutical litigation can provide valuable guidance and increase the likelihood of a favorable outcome.
Conclusion
The unfolding Oxbryta litigation underscores the critical importance of drug safety and the responsibility of pharmaceutical companies to provide transparent and comprehensive information about potential risks. As legal proceedings continue, affected individuals are encouraged to seek legal counsel to explore their options for compensation and to hold manufacturers accountable for alleged negligence.